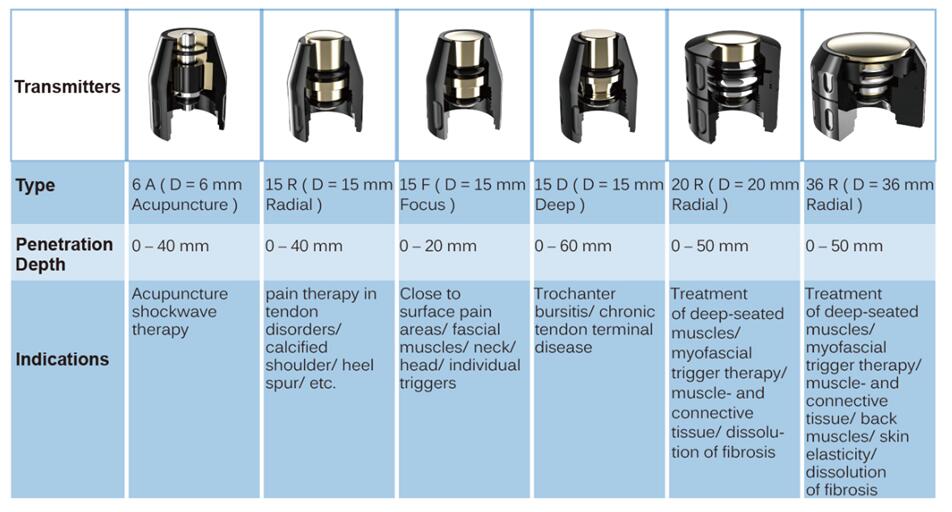
frequency: 1 - 22hz
pressure: 1.0 - 5.0 bar
channel: 1 channel
interface: 10" touch screen
integrated design---main unit and trolley combined
user-friendly interface with large touch screen
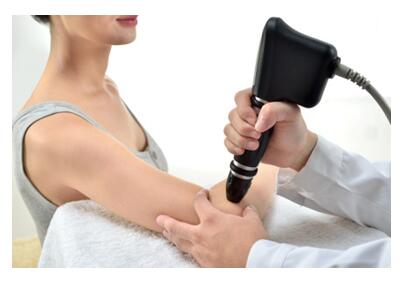
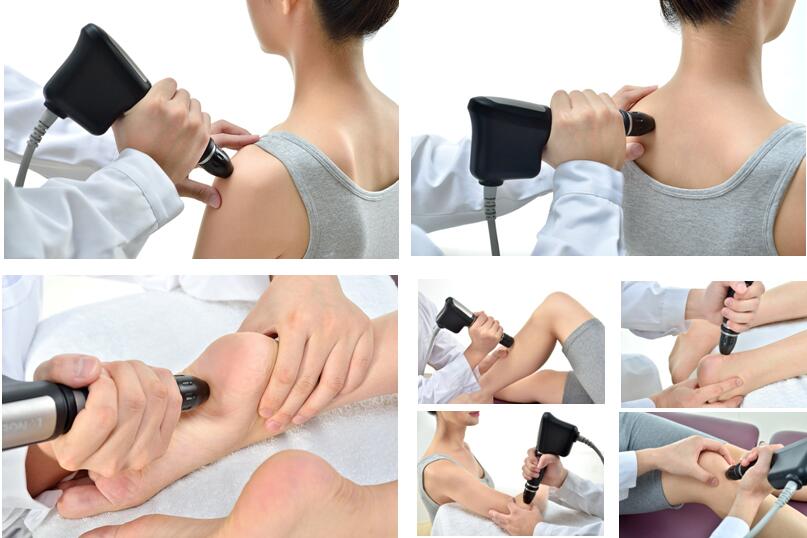
treatment guidance with large & clear photos
pre-set protocols for most popular clinical use
user-defined protocols
patient database with vas score
pdf file of vas score output supported
software updating by usb port supported
specific indications on this site are not approved by the fda in the usa.
for indications that are cleared through the usa fda please refer to our usa webpage at